The Nose Knows
INTRODUCTION:
Chemistry is a great vehicle for building students' respect for their bodies and to motivate them to take greater care of themselves.
CHEMICAL CONCEPTS:
Synthesis of Esters
Chemical basis of odours
MATERIALS:
Salicylic acid
Anthranilic Acid
Pure methanol
Concentrated sulfuric acid
Test tubes and stoppers
400 mL beakers
PROCEDURE:
Interesting esters can be made easily with an overnight experiment.
Half of the class should use salicylic acid and half should use anthranilic acid.
Have students mix a "scoopful" (about 2 g) of salicylic acid powder with about 25 mL of pure methyl alcohol in a labeled 125 mL Erlenmeyer flask. When the solid is dissolved, they should bring it to you to have you add drop-wise, about one mL of conc. sulfuric acid.
They should then stopper the flask and store on a shelf in a secure cupboard.
The next day, instruct the class about care with odours and show them how to waft the odour toward their noses. Also, be sure to remind them that the sulfuric acid acts as a catalyst and thus is still in the liquid! This should pre-empt any requests about tasting the product.
Then have them carefully pour the contents of their flasks into a 400-mL beaker half-filled with warm (not hot) water, and sniff the results.
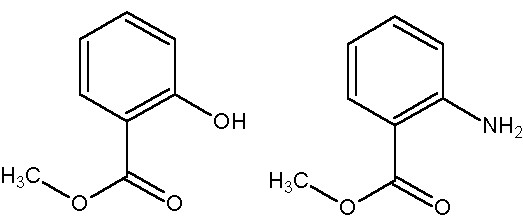
Some will smell the pungent odour of wintergreen, but the ones using anthranilic acid will smell "grape". The molecules differ by only one group. Hydroxyl gives wintergreen. Amino gives grape.
SAFETY PRECAUTIONS:
[These suggestions are NOT intended to be a complete review of all the safety issues involved with this activity. Professional judgement and practices are essential. If you are unsure of the safety precautions that should be taken, seek experienced assistance.]
The major hazard here is the handling of concentrated sulfuric acid. The easiest solution is for the teacher to add the sulfuric acid to the test tube for the students.
It must be added drop-wise to avoid overheating and decomposition of the organic acids. Once the acid is in the test-tube, it is dilute enough that any spillage can be washed off before any skin damage will occur.
Of course, safety goggles are very important in all stages of this experiment.
DISPOSAL:
Flinn #24b. Neutralize the sulphuric acid with sodium carbonate in a fume hood. The volatile esters will be carried off with the CO2 gas.
Flinn #24a also applies.
DISCUSSION:
A GC-IR spectrometer costs thousands of dollars. Your students each have a very costly chemical analyzer right between their eyes!

Methyl Salicylate........................ ..... Methyl Anthranilate
(wintergreen) ............................................ (grape)
Materials available from Flinn Scientific:
Catalog No. & Description
S0001 Salicylic acid
A0304 Anthranilic acid
M0054 Methyl alcohol
S0228 Sulfuric acid (18M)